The advent of the semiconductor has revolutionised our lives, since it is the basis of all integrated circuits and microprocessors.
To distinguish between the electrical properties of materials we can group them into three sections:
(a) conductors,
(b) semiconductors and
(c) insulators.
You are probably aware of many conductors and insulators such as copper and rubber; semiconductors include materials such as silicon, germanium, carbon, selenium, gallium arsenide, lead sulphide.
The important difference between conductors, semiconductors and insulators lies in the number of free electrons present in the material. Perhaps the best way to consider the differences between them is to use the band theory of solids.
As you may know, electrons in an individual atom are restricted to well-defined energy levels and energy changes within the atom only take place between one level and another.
In a solid the atoms are linked together and the electrons can occupy a whole series of energy levels grouped into bands (see Figure 1). The difference in energy between levels within the band is very small compared with the energy gap between the bands. The electrical differences between one type of solid and another lie in the different arrangements of the bands.
The band structures of a conductor, semiconductor and insulator are shown in Figure 2.
Conductors
In a conductor the valence band is full of electrons, while the conduction band has some free electrons and many empty energy levels. The addition of a very small amount of energy will allow electrons to move within the conduction band, some rising to a higher level and others returning to lower levels. This movement of electrons is electrical conduction.In some conductors the valence band and the con- duction band actually overlap. This effectively gives a partly filled top band.
Intrinsic semiconductors
We will deal first with the intrinsic semiconductor. This is a material that is a semiconductor 'in its own right' - nothing has been added to it.In the intrinsic semiconductor the valence band is full once more, but the conduction band is empty at very low temperatures. However, the energy gap between the two bands is so very small that electrons can jump across it by the addition of thermal energy alone or even light energy of a suitable wavelength. In other words, heating the specimen or shining a light on it maybe sufficient to cause electrical conduction. The conductivity increases with temperature as more and more electrons are liberated. Semiconductors therefore have negative temperature coefficients of resistance.
For germanium the energy gap is 0.66 eV and for silicon it is 1.11 eV at 27 oC. When an electron jumps to the conduction band it leaves behind it a space or hole in the valence band. This hole is effectively positive and since an electron can jump into it from another part of the valence band it is as if the hole itself was moving! Conduction can take place either by negative electrons moving within the conduction band or by positive holes moving within the valence band.
A semiconductor may be thought of as similar to an almost full multi-storey car park, the cars representing the electrons and the spaces the holes (no cars are allowed to enter or leave the car park, however, only to drive round within it!). (Figure 3)
If this idea of holes seems odd to you, think of a pile of earth and the hole in the road from which it came. Both the pile (electron) and the hole (hole) have a physical effect on you if you run into them on a bike! Conduction by positive holes is rather like workmen digging up a road; in a way, they are only moving a hole from one place to another.
Insulators
In the insulator the valence band is full once again, but in these substances the energy gap between this and the empty conduction band is very large. It would take a great deal of energy to make an electron jump the gap and to cause the insulator to break down. At very high temperatures or under very large electric fields breakdown will occur, and like semiconductors the greater the temperature the greater the conduction. Insulators, like semiconductors, have negative temperature coefficients of resistance.
Student investigationThe thermistor is a semiconductor device whose resistance changes markedly with temperature.
Using a negative temperature coefficient thermistor set up the circuit shown in Figure 4 and record values of the current and voltage for a range of temperatures.
RELATED VIDEO

 The field-effect transistor (FET) is a transistor that uses an electric field to control the shape and hence the conductivity of a channel of one type of charge carrier in a semiconductor material. FETs are sometimes called unipolar transistors to contrast their...
The field-effect transistor (FET) is a transistor that uses an electric field to control the shape and hence the conductivity of a channel of one type of charge carrier in a semiconductor material. FETs are sometimes called unipolar transistors to contrast their...
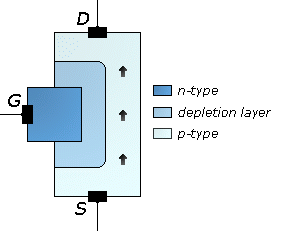 The junction gate field-effect transistor (JFET or JUGFET) is the simplest type of field-effect transistor. It can be used as an electronically-controlled switch or as a voltage-controlled resistance. Electric charge flows through a semiconducting channel between...
The junction gate field-effect transistor (JFET or JUGFET) is the simplest type of field-effect transistor. It can be used as an electronically-controlled switch or as a voltage-controlled resistance. Electric charge flows through a semiconducting channel between...