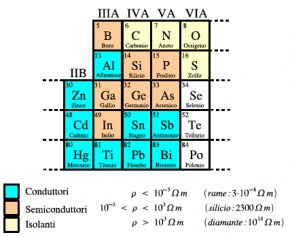
 There is an extremely straightforward explanation for this. We have metals - the conductors of electricity and non-metals, which are the opposite. Semi-conductors are the elements which lie in-between.
There is an extremely straightforward explanation for this. We have metals - the conductors of electricity and non-metals, which are the opposite. Semi-conductors are the elements which lie in-between.
As seen in the above diagram, the semiconductors lie in an area between the metals and non-metals.
Now, before we proceed to the heart of this question, we must understand why metals conduct electricity. Every atom has an outer band of electrons, known as the valence band. In metals, the electrons from this valence band are not confined to the atom and are free to move throughout the metal lattice. It is this "sea of electrons" which makes conduction possible. It is exactly the opposite in non-metals, where the electrons are held tightly.
Semi-conductors act as non-metals at low temperatures - the electrons are trapped within the atom. As the temperature of the semi-conductor is increased, the electrons in the valence band gain sufficient energy to escape from the confines of their atoms. As a result, in higher temperatures, a semi-conductor's valence electrons are free = conduction results, resistivity decreases.
We call the energy required for an electron to escape - the "band-gap" of a semi-conductor.
Higher the bandgap of a semi-conductor - more the energy needed to convert it into a conductor. The bandgap of Germanium is 0.67 eV, silicon at 1.1 eV. What can be deduced is that it takes a lower temperature to convert Germanium to a conductor than silicon.
RELATED VIDEO


